In a remarkable breakthrough in cancer treatment, researchers from Mass General Cancer Center, in collaboration with neurosurgeons from the same institution, have developed a pioneering CAR-T therapy variant named CARv3-TEAM-E T cells. This new therapy has shown astonishing results in a clinical trial involving three patients with recurrent glioblastoma, a particularly deadly form of brain cancer.
Understanding Glioblastoma
Glioblastoma is an aggressive type of cancer that begins in the cells of the brain or spinal cord. It is known for its rapid growth and its ability to invade and destroy healthy brain tissue. The prognosis for this cancer is generally poor, making the recent findings all the more significant.
The Clinical Trial

The phase one clinical trial, conducted between March and July 2023, involved three patients: a 57-year-old woman and two men aged 72 and 74. These patients were selected due to their recurring cases of glioblastoma, each having faced bleak outlooks.
Innovations in Treatment: CARv3-TEAM-E T Cells
The treatment utilized in the trial, CARv3-TEAM-E T cells, represents an innovative approach to CAR-T therapy. Traditional CAR-T therapy, which has been effective in treating blood cancers, involves reengineering a patient’s T cells to target cancer more effectively. However, applying this method to solid tumors like glioblastoma has been challenging due to the heterogeneity of the tumor cells.
To enhance the efficacy of CAR-T therapy in solid tumors, researchers combined it with T-cell engaging antibody molecules (TEAMs). These modified CAR T cells are engineered to target both the epidermal growth factor receptor (EGFR) variant III and the wild-type EGFR protein. This dual targeting is crucial for attacking the mixed cell populations within glioblastoma tumors.
Dramatic Results
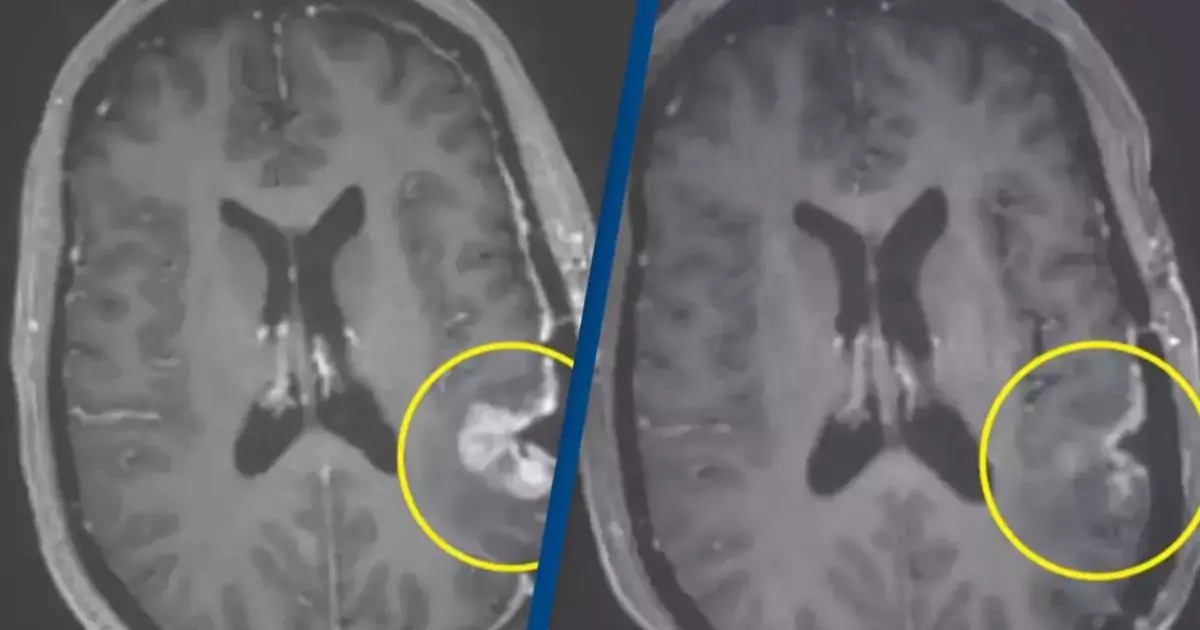
The trial’s results were nothing short of spectacular. MRI scans conducted after the treatments showed dramatic reductions in tumor size:
- The 57-year-old woman exhibited near-complete tumor regression just five days after a single infusion of the therapy.
- The 72-year-old man showed an 18.5% reduction in tumor size two days after treatment, which increased to a 60.7% reduction by day 69.
- The 74-year-old man experienced a significant decrease in EGFRvIII and EGFR protein levels, eventually becoming undetectable in his blood and cerebrospinal fluid.
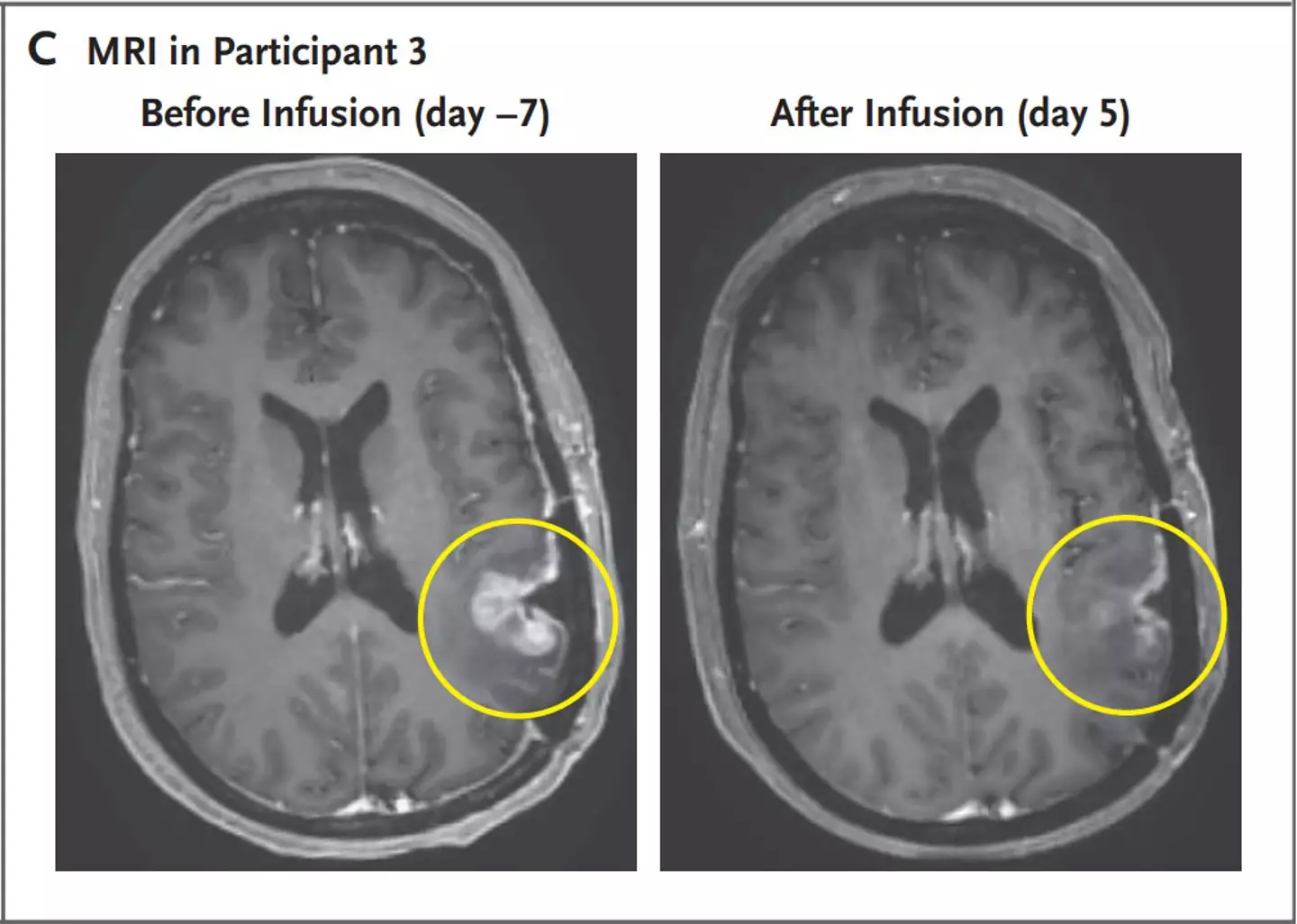
Patient Response and Safety
All three patients tolerated the treatment well, though they experienced some expected side effects such as fevers and altered mental states shortly after infusion. These effects were considered manageable, falling within grade three or dose-limiting toxic effects, which are within acceptable limits for such groundbreaking treatments.
Expert Reactions
Neurosurgeon Bryan Choi, a key figure in the development of this therapy, emphasized the potential of the CAR-T platform to treat solid tumors effectively by combining multiple therapeutic strategies. Marcela Maus, another lead researcher, remarked on the promising nature of these results, viewing them as a significant step toward developing a cure for glioblastoma.
Future Implications
The success of this trial marks a pivotal moment in the treatment of glioblastoma and possibly other solid tumors. The results, recently published in The New England Journal of Medicine, are a beacon of hope for patients suffering from this formidable cancer. While the journey towards a cure continues, the progress made by the Mass General team is a testament to the potential of innovative cancer therapies to significantly alter the course of previously untreatable diseases.